- 首页
- 关于我们
概况介绍 研究方向 人才培养 委员会/committee
- 员工团队
特聘专家 主要领导 行政团队 技术团队 PI团队- 科研平台
中心实验室 实验动物中心 生物样本库- 最新资讯
- 首页
- 关于我们
概况介绍 研究方向 人才培养 委员会/committee
- 员工团队
特聘专家 主要领导 行政团队 技术团队 PI团队- 科研平台
中心实验室 实验动物中心 生物样本库- 最新资讯
Single-cell RNA sequencing study of retinal immune regulators identified CD47 and CD59a expression in photoreceptors-Implications in subretinal immune regulation发布日期: 2024-09-03
Abstract
The neuroretina is protected by its own defense system, that is microglia and the complement system. Under normal physiological conditions, microglial activation is tightly regulated by the neurons although the underlying mechanism remains elusive. Using published single-cell RNA sequencing data sets, we found that immune regulatory molecules including CD200, CD47, CX3CL1, TGFβ, and complement inhibitor CD59a are expressed by various retinal neurons. Importantly, we found that photoreceptors express higher levels of CD47 and CD59a, which was further confirmed in cultured 661W cells, WERI-Rb1 cells, and microdissected photoreceptors from human eyes. The expression of CD59a mRNA in 661W cells was upregulated by TNFα and hypoxia, whereas LPS, hypoxia, and IL-4 upregulated CD47 mRNA expression in 661W cells. Immunofluorescence staining detected strong CD59a immunoreactivity in the outer nuclear layer, inner/outer segments, and discrete staining in ganglion cell layer (GCL), inner plexiform layer (IPL), and outer plexiform layer. The expression of CD59a in photoreceptors was increased in the detached retina, but decreased in retinas from experimental autoimmune uveoretinitis (EAU) mice. In EAU retina, CD59a was highly expressed by active immune cells. CD47 was detected in GCL, IPL, and inner nuclear layer and some photoreceptors. The expression of CD47 in photoreceptors was also increased in the detached retina but decreased in EAU retina. In a coculture system, 661W enhanced arginase-1 and reduced IL-6 mRNA expression in BV2 microglial cells. Our results suggest that photoreceptors express immune regulatory molecules and may have the potential to regulate immune activation in the outer retina/subretinal space under pathophysiological conditions.
相关文章-
High myopia is a global health concern, often leading to degenerative retinal ch...2025-10-23
-
近期,爱尔眼科研究所徐和平教授团队在眼病机理方面研究取得了多项令人瞩目的成果,为了解致盲性视网膜疾病的发病机制与治疗新靶点的发现带来了希望。2025-07-21
-
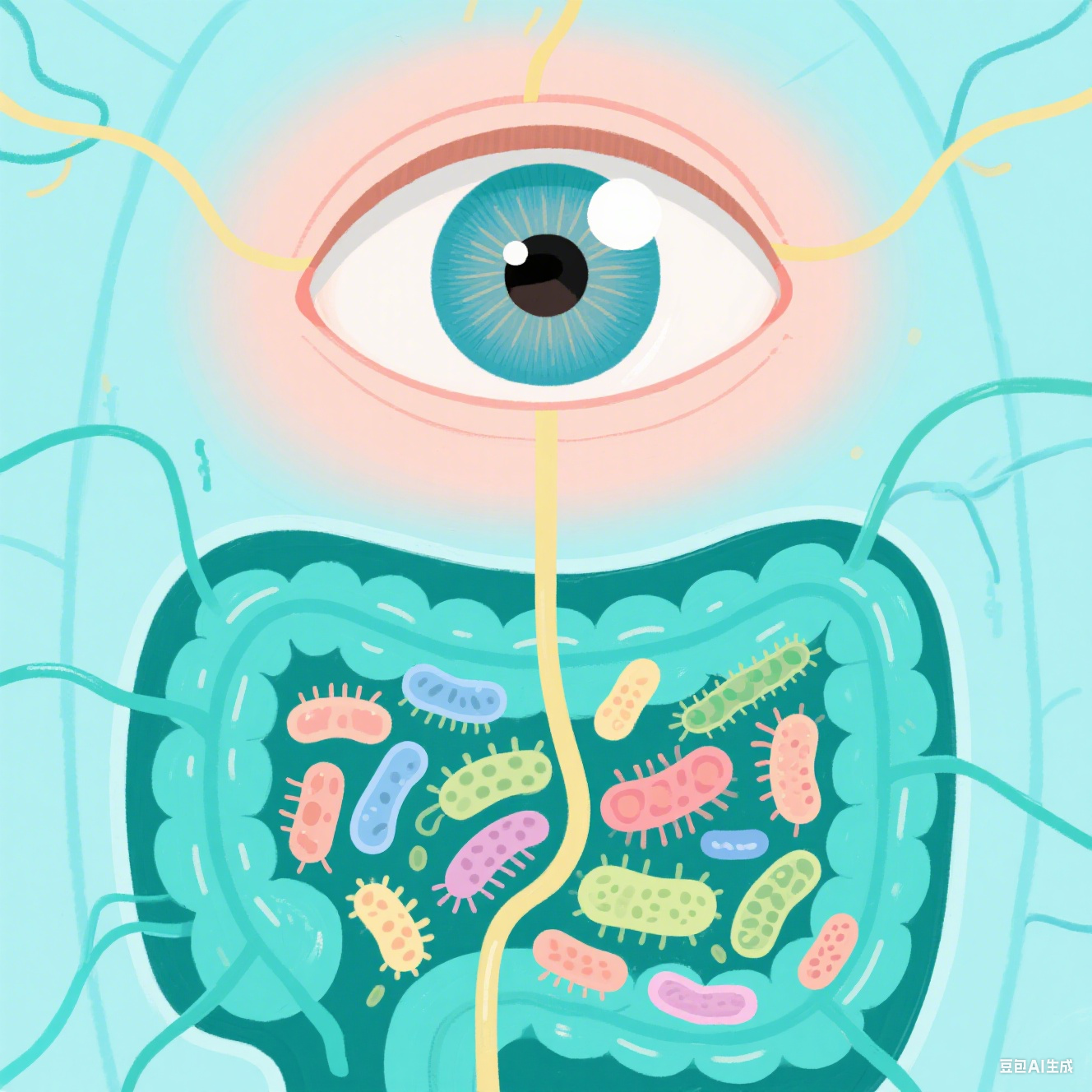
AbstractPURPOSE. The purpose of this study was to understand how the gut microbi...2025-05-09
-
Inherited retinal diseases (IRDs) can induce severe sight-threatening retinal de...2024-09-11
-
Corneal neovascularization (CNV) is a condition that can severely adversely affe...2024-09-05
-
Diabetic retinopathy (DR) is a common complication of diabetes and has a high pr...2024-09-04
-
Diabetic retinopathy (DR) development is associated with disturbances in the gut...2024-09-04
-
The three-dimensional (3D) retinal organoids (ROs) derived from human induced pl...2024-09-04
-
Tissue injury is a common clinical problem, which may cause great burden on pati...2024-09-04
-
Corneal nerve wounding often causes abnormalities in the cornea and even blindne...2024-09-04
-
Pre-mRNA processing factors (PRPFs) are vital components of the spliceosome and ...2024-09-04
-
Propagating large amounts of human corneal stromal cells (hCSCs) in vitro while ...2024-09-04
-
This study aimed to determine the effect of pinacidil, a nonselective KATP chann...2024-09-04
-
In vertebrates, most somatosensory pathways begin with the activation of dorsal ...2024-09-04
-
The pathogenesis of type 2 diabetes mellitus (T2DM) is commonly associated with ...2024-09-04
-
Corneal fibroblast can be transformed into corneal myofibroblasts by TGF-β1.2024-09-04
-
Age-related macular degeneration (AMD) is a common vision-threatening disease. T...2024-09-04
-
Suspended spheroid culture using ultralow attachment plates (ULAPs) is reported ...2024-09-04
-
Purpose: Retinal inflammation is involved in the pathogenesis of several retinal...2024-09-03
-
Aging is a risk factor for multiple retinal degeneration diseases. Entraining br...2024-09-03
-
Angiographically silent cystoid macular edema (CME) is a rare complication from ...2024-09-03
-
The neuroretina is protected by its own defense system, that is microglia and th...2024-09-03
-
Purpose: We performed a bioinformatic transcriptome analysis to determine the al...2024-09-03
-
Previous research has shown that CXCR5−/− mice develop retinal degeneration (RD)...2024-09-03
-
Purpose: To investigate the levels of matrix metalloproteinases (MMPs) in aqueou...2024-09-03
-
In vitro generation of a functional retinal pigment epithelium (RPE) monolayer s...2024-09-03
-
We have established an induced pluripotent stem (iPS) cell line using urine-deri...2024-09-03
-
Purpose: The purpose of this study is to investigate the potential therapeutic b...2024-09-03
-
We report the human induced pluripotent stem cell line (iPSC) CSUASOi002-A, gene...2024-09-03
-
Background Central serous chorioretinopathy (CSC) is a widespread retinal disord...2024-09-03
-
Glaucoma is characterized by progressive, irreversible damage to the retinal gan...2024-09-03
-
Subarachnoid space (SAS) around optic nerve can be visible with swept-source opt...2024-09-03
-
Diabetic eye disease refers to a group of eye complications that occur in diabet...2023-05-01
- 员工团队
- 员工团队