- 首页
- 关于我们
概况介绍 研究方向 人才培养 委员会/committee
- 员工团队
特聘专家 主要领导 行政团队 技术团队 PI团队- 科研平台
中心实验室 实验动物中心 生物样本库- 最新资讯
- 首页
- 关于我们
概况介绍 研究方向 人才培养 委员会/committee
- 员工团队
特聘专家 主要领导 行政团队 技术团队 PI团队- 科研平台
中心实验室 实验动物中心 生物样本库- 最新资讯
Characteristics of neural growth and cryopreservation of the dorsal root ganglion using three-dimensional collagen hydrogel culture versus conventional culture发布日期: 2024-09-04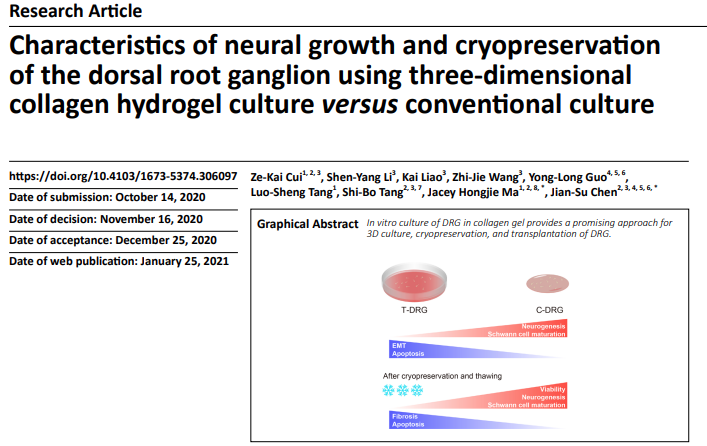
Abstract
In vertebrates, most somatosensory pathways begin with the activation of dorsal root ganglion (DRG) neurons. The development of an appropriate DRG culture method is a prerequisite for establishing in vitro peripheral nerve disease models and for screening therapeutic drugs. In this study, we compared the changes in morphology, molecular biology, and transcriptomics of chicken embryo DRG cultured on tissue culture plates (T-DRG) versus three-dimensional collagen hydrogels (C-DRG). Our results showed that after 7 days of culture, the transcriptomics of T-DRG and C-DRG were quite different. The upregulated genes in C-DRG were mainly related to neurogenesis, axon guidance, and synaptic plasticity, whereas the downregulated genes in C-DRG were mainly related to cell proliferation and cell division. In addition, the genes related to cycles/pathways such as the synaptic vesicle cycle, cyclic adenosine monophosphate signaling pathway, and calcium signaling pathway were activated, while those related to cell-cycle pathways were downregulated. Furthermore, neurogenesis- and myelination-related genes were highly expressed in C-DRG, while epithelial–mesenchymal transition-, apoptosis-, and cell division-related genes were suppressed. Morphological results indicated that the numbers of branches, junctions, and end-point voxels per C-DRG were significantly greater than those per T-DRG. Furthermore, cells were scattered in T-DRG and more concentrated in C-DRG, with a higher ratio of 5-ethynyl-2′-deoxyuridine (EdU)-positive cells in T-DRG compared with C-DRG. C-DRG also had higher S100 calcium-binding protein B (S100B) and lower α-smooth muscle actin (α-SMA) expression than T-DRG, and contained fewer terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)-positive cells after 48 hours of serum starvation. After cryopreservation, C-DRG maintained more intact morphological characteristics, and had higher viability and less TUNEL-positive cells than T-DRG. Furthermore, newly formed nerve bundles were able to grow along the existing Schwann cells in C-DRG. These results suggest that C-DRG may be a promising in vitro culture model, with better nerve growth and anti-apoptotic ability, quiescent Schwann cells, and higher viability. Results from this study provide a reference for the construction, storage, and transportation of tissue-engineered nerves. The study was approved by the Ethics Committee of Aier School of Ophthalmology, Central South University, China (approval No. 2020-IRB16), on March 15, 2020.
相关文章-
Generation of vascularized retinal organoids containing microglia based on a PDMS microwell platformAbstractRetinal organoids (ROs) offer a biomimetic in vitro model for inves...2025-10-24
-
近期,爱尔眼科长沙医学中心陈建苏教授、唐仕波教授、崔泽凯副教授团队在角膜损伤修复和视网膜疾病治疗方面研究取得了多项令人瞩目的成果,为了解致盲性角膜和视网膜疾病的...2025-08-04
-
近日,由中国医师协会眼科医师分会遗传眼病学组牵头制定的《X连锁视网膜劈裂症临床诊疗的中国专家共识(2025)》(以下简称《共识》)正式发布。X连锁视网膜劈裂症是...2025-07-07
-
AbstractKeratoconus (KC) is a prevalent ectatic corneal disease influenced by co...2025-05-06
-
AbstractBackground Recent advances in clinical trials have involved the transpla...2025-05-06
-
Inherited retinal diseases (IRDs) can induce severe sight-threatening retinal de...2024-09-11
-
Corneal neovascularization (CNV) is a condition that can severely adversely affe...2024-09-05
-
Diabetic retinopathy (DR) is a common complication of diabetes and has a high pr...2024-09-04
-
Diabetic retinopathy (DR) development is associated with disturbances in the gut...2024-09-04
-
The three-dimensional (3D) retinal organoids (ROs) derived from human induced pl...2024-09-04
-
Tissue injury is a common clinical problem, which may cause great burden on pati...2024-09-04
-
Corneal nerve wounding often causes abnormalities in the cornea and even blindne...2024-09-04
-
Pre-mRNA processing factors (PRPFs) are vital components of the spliceosome and ...2024-09-04
-
Propagating large amounts of human corneal stromal cells (hCSCs) in vitro while ...2024-09-04
-
This study aimed to determine the effect of pinacidil, a nonselective KATP chann...2024-09-04
-
In vertebrates, most somatosensory pathways begin with the activation of dorsal ...2024-09-04
-
The pathogenesis of type 2 diabetes mellitus (T2DM) is commonly associated with ...2024-09-04
-
Corneal fibroblast can be transformed into corneal myofibroblasts by TGF-β1.2024-09-04
-
Age-related macular degeneration (AMD) is a common vision-threatening disease. T...2024-09-04
-
Suspended spheroid culture using ultralow attachment plates (ULAPs) is reported ...2024-09-04
-
本申请涉及细胞和器官培养技术领域,公开了一种三维类器官灌流培养器具,包括:细胞培养室,细胞培养室用于盛放培养基;多个换液槽,设于细胞培养室,用于更换细胞培养室中...2024-09-04
-
Purpose: Retinal inflammation is involved in the pathogenesis of several retinal...2024-09-03
-
Aging is a risk factor for multiple retinal degeneration diseases. Entraining br...2024-09-03
-
Angiographically silent cystoid macular edema (CME) is a rare complication from ...2024-09-03
-
The neuroretina is protected by its own defense system, that is microglia and th...2024-09-03
-
Purpose: We performed a bioinformatic transcriptome analysis to determine the al...2024-09-03
-
Previous research has shown that CXCR5−/− mice develop retinal degeneration (RD)...2024-09-03
-
Purpose: To investigate the levels of matrix metalloproteinases (MMPs) in aqueou...2024-09-03
-
In vitro generation of a functional retinal pigment epithelium (RPE) monolayer s...2024-09-03
-
We have established an induced pluripotent stem (iPS) cell line using urine-deri...2024-09-03
-
Purpose: The purpose of this study is to investigate the potential therapeutic b...2024-09-03
-
We report the human induced pluripotent stem cell line (iPSC) CSUASOi002-A, gene...2024-09-03
-
Background Central serous chorioretinopathy (CSC) is a widespread retinal disord...2024-09-03
-
Glaucoma is characterized by progressive, irreversible damage to the retinal gan...2024-09-03
-
Subarachnoid space (SAS) around optic nerve can be visible with swept-source opt...2024-09-03
-
Diabetic eye disease refers to a group of eye complications that occur in diabet...2023-05-01
- 员工团队
- 员工团队